Новые технологические подходы к созданию препарата уриказы с низкой иммуногенностью и улучшенными терапевтическими свойствами
У человека и высших приматов отсутствует уриказа — фермент, окисляющий мочевую кислоту, и как результат для людей характерен высокий уровень мочевой кислоты в сыворотке. У некоторых людей концентрация мочевой кислоты повышается выше порога растворимости, что приводит к образованию кристаллов солей мочевой кислоты в суставах и развитию острого воспаления, вызывающего сильную боль. Это состояние известно, как подагра. В лечении тяжелой подагры для снижения уровня мочевой кислоты в крови применяют инъекции нечеловеческой уриказы. Krystexxa® — это препарат гиперпегилированной химерной уриказы медвежьего павиана, который применяется при хронической рефрактерной подагре, В 91% случаев введение препарата сопровождается развитием иммунных реакций, в том числе инфузионных реакций (26%) и анафилаксии (6,5%), что ограничивает применение и эффективность препарата. Был использован новый подход для создания препарата с улучшенными свойствами: экспрессия растворимой формы соединения, растворимость при нейтральных значениях pH, высокий уровень экспрессии у E. coli, стабильность в широком диапазоне температур и высокая активность. Для конструирования белка и ограничения его эффектов были объединены более 200 различных последовательностей, кодирующих уриказы. Был найден один основной кандидат с низким потенциалом иммуногенности в > 200 человеческих донорских образцах с различными гаплотипами HLA. Были сконструированы цистеины для специфического пегилирования основной последовательности, и, по результатам исследований, эффективность пегилирования составила > 95%. Пегилированная уриказа сохраняет ферментную активность in vitro при нейтральных значениях рН, в человеческой сыворотке и in vivo (у крыс и собак) и обладает увеличенным периодом полувыведения. У собак после однократной подкожной инъекции препарата уровень мочевой кислоты в сыворотке снизился на 85%. Возможно, эта пегилированная неиммуногенная уриказа принесет существенную пользу больным подагрой.

PLoS One. 2016 Dec 21;11(12):e0167935. doi: 10.1371/journal.pone.0167935. eCollection 2016.
PMID: 28002433. DOI: 10.1371/journal.pone.0167935
Andrew C. Nyborg , Chris Ward, Anna Zacco, Benoy Chacko †, Luba Grinberg, James C. Geoghegan, Ryan Bean, Michaela Wendeler, Frank Bartnik, Ellen O’Connor, Flaviu Gruia, Vidyashankara Iyer, Hui Feng, Varnika Roy, Mark Berge, Jeffrey N. Miner, David M. Wilson, Dongmei Zhou, Simone Nicholson, Clynn Wilker, Chi Y. Wu, Susan Wilson, Lutz Jermutus, Herren Wu, David A. Owen, Jane Osbourn, Steven Coats, Manuel Baca
![]()
A Therapeutic Uricase with Reduced Immunogenicity Risk and Improved Development Properties
Abstract
Humans and higher primates are unique in that they lack uricase, the enzyme capable of oxidizing uric acid. As a consequence of this enzyme deficiency, humans have high serum uric acid levels. In some people, uric acid levels rise above the solubility limit resulting in crystallization in joints, acute inflammation in response to those crystals causes severe pain; a condition known as gout. Treatment for severe gout includes injection of non-human uricase to reduce serum uric acid levels. Krystexxa® is a hyper-PEGylated pig-baboon chimeric uricase indicated for chronic refractory gout that induces an immunogenic response in 91% of treated patients, including infusion reactions (26%) and anaphylaxis (6.5%). These properties limit its use and effectiveness. An innovative approach has been used to develop a therapeutic uricase with improved properties such as: soluble expression, neutral pH solubility, high E. coli expression level, thermal stability, and excellent activity. More than 200 diverse uricase sequences were aligned to guide protein engineering and reduce putative sequence liabilities. A single uricase lead candidate was identified, which showed low potential for immunogenicity in >200 human donor samples selected to represent diverse HLA haplotypes. Cysteines were engineered into the lead sequence for site specific PEGylation and studies demonstrated >95% PEGylation efficiency. PEGylated uricase retains enzymatic activity in vitro at neutral pH, in human serum and in vivo (rats and canines) and has an extended half-life. In canines, an 85% reduction in serum uric acid levels was observed with a single subcutaneous injection. This PEGylated, non-immunogenic uricase has the potential to provide meaningful benefits to patients with gout.
A Therapeutic Uricase with Reduced Immunogenicity Risk and Improved Development Properties
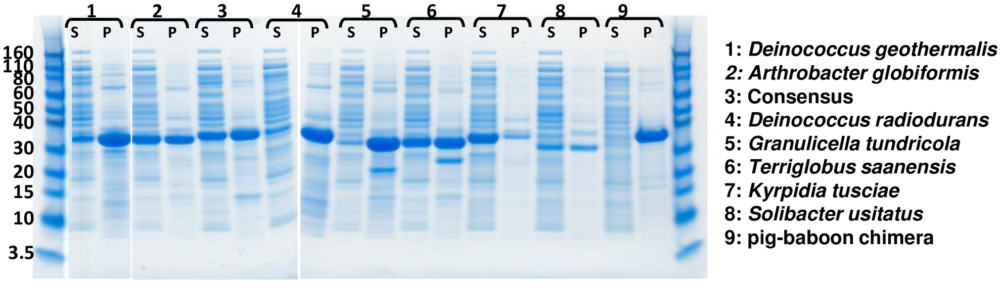
As shown in Fig 1, most uricases were present at high level in the insoluble (P) material. The pig-baboon chimera appears to express almost entirely in the insoluble (P) fraction (Lane 9). Cytosolic soluble (S) expression was considered favorable. http://dx.doi.org/10.1371/journal.pone.0167935.g001
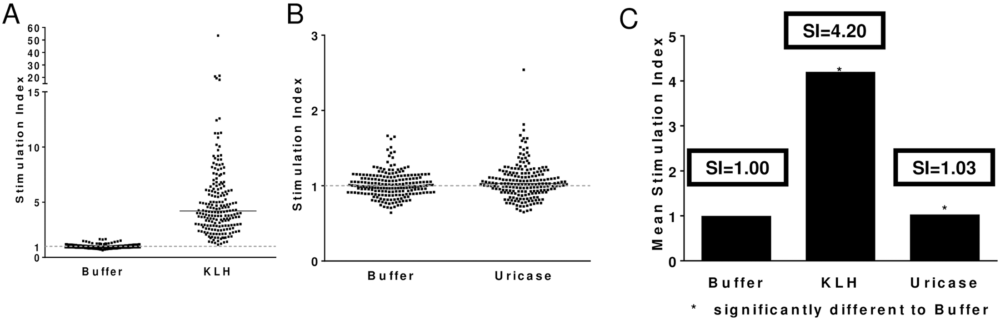
The Epibase® assay is a human PBMC T-cell immunogenicity assay used to assess “immunogenicity risk”. In this assay, PBMC samples from 202 normal donors were used to screen the T-cell immunogenicity of a modified Arthrobacter globiformis uricase candidate relative to a negative control (buffer) and a positive control (KLH). The 202 donors were selected to represent HLA-DRB1 frequencies observed in a Caucasian population (S4 Fig). Stimulation indices (SI) values describe the ratio of proliferating CD3+CD4+ T-cell in antigen treated versus untreated wells. SI values >2 are considered positive which is supported by a p-value <0.05. A) Shows the individual SI values for negative control—0/202 donor samples (0%) responded with SI>2.0; and positive control—181/202 donors (91%) responded with a SI>2.0. B) Shows the individual SI values for negative control and modified uricase candidate—1/202 donor samples responded with a SI>2.0. C) Shows the mean stimulation index for all donor samples for buffer (SI = 1.0), KLH SI = 4.2 and modified Arthrobacter globiformis (SI = 1.03). http://dx.doi.org/10.1371/journal.pone.0167935.g006
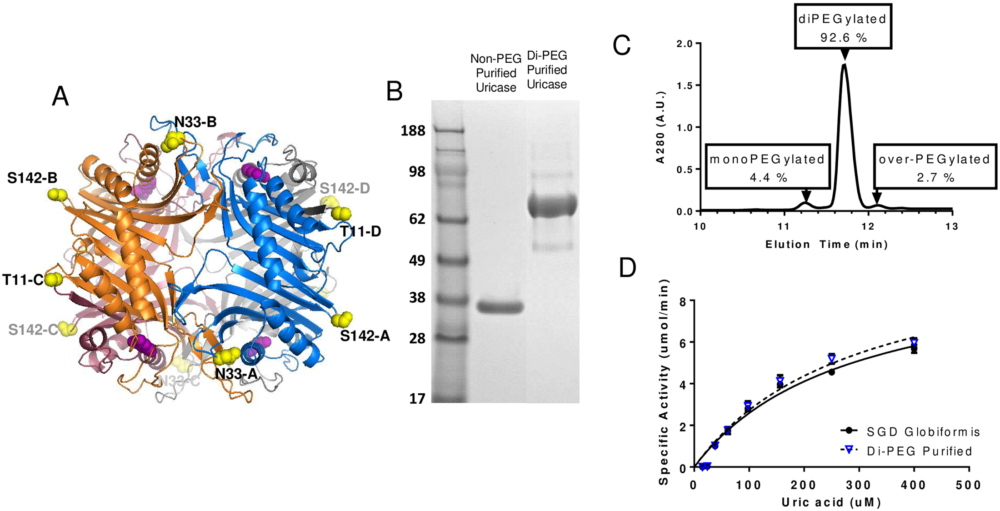
(A) Shows the three dimensional solvent accessible sites within the tetrameric crystal structure of Arthrobacter globiformis uricase [1]. Each uricase monomer subunit of the tetrameric enzyme is shown in a different color, and residues selected for substitution with Cys (T11, N33, S142) are shown in yellow. (B) SDS-PAGE analysis of di-PEGylated and non-PEGylated uricase suggests that the di-PEGylated material runs at a much higher molecular weight relative to the non-PEGylated material. (C) A reverse-phase chromatography analysis of the di-PEGylated material suggests that 92.6% of the material is di-PEGylated, 4.4% mono-PEGylated, 0% unreacted and 2.7% over-PEGylated. (D) Demonstrates that the activity of di-PEGylated uricase is comparable to non-PEGylated uricase. http://dx.doi.org/10.1371/journal.pone.0167935.g007
